Sodium-Ion Battery-Charge Battery In Seconds-24
Sodium-Ion Battery
Due to the cheap and plentiful nature of sodium supplies,Sodium-Ion Battery-Charge Battery In Seconds has become one of the most exciting new players in the energy storage market. LIBs may be replaced by SIBs. Research into SiBs has been rapid in an effort to capitalize on their benefits for a range of uses, from grid-scale energy storage to portable electronics, as a result of the growing need for effective and sustainable energy storage solutions.
After making a ground-breaking discovery in sodium-ion battery technology, researchers from the Korea Advanced Institute of Science and Technology (KAIST) have advanced the field of energy storage. Under the direction of Professor Jeung Ku Kang, the team’s inventiveness promises to change the portable power market.
Follow our Digiknowledge.co.in page for the latest updates about bikes, cars, sports, lifestyle, and many more.
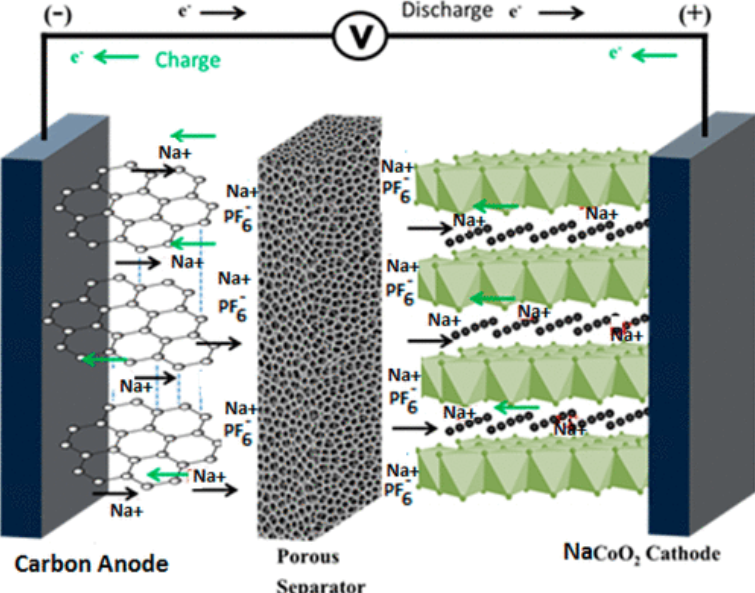
What are the components of a Sodium-Ion Battery?
Key components that work in harmony to store and release energy are as follows:.
- Cathode, or positive electrode:
Within the battery, the cathode is where energy is stored. It is usually made up of layered materials that contain sodium, which is important since it affects the battery’s energy density, capacity, and cell voltage.
During the charge and discharge cycles, these layered materials go through reversible chemical reactions that enable the storage and release of sodium ions.
- Anode, or negative electrode:
By aiding in the passage of sodium ions during battery operation, the anode supports the cathode. Hard carbons or intercalation compounds, which offer sodium ions a stable host structure, are frequently used in its construction.
The anode is usually covered with a layer of carbon to improve conductivity and reduce unwanted side reactions, hence increasing lifetime and performance.
- Separator:
The separator, which functions as a physical barrier between the cathode and anode, is an essential part that lets sodium ions flow while preventing short circuits.
The separator reduces the possibility of electrical contact while facilitating ion passage between the electrodes since it is made of a porous substance.
In order for sodium ions to go from the cathode to the anode and complete the circuit during battery operation, the electrolyte is an essential medium.
Both aqueous and non-aqueous electrolytes—which both comprise sodium salt dissolved in a solvent mixture—can be used, depending on the particular application and design factors.
The performance of the battery is mostly dependent on the composition and characteristics of the electrolyte, including its cycle stability, rate capacity, and safety qualities.
This innovation is based on the underlying functional and design similarities between lithium-ion and sodium-ion batteries. For example, in sodium-ion batteries, sodium ions take the place of lithium ions in the cathode, and sodium salts are used in the electrolyte, which facilitates the transfer of charge between the electrodes of the battery, instead of lithium salts.
As a viable substitute for conventional lithium-ion batteries, sodium-ion batteries provide a number of significant benefits. Because sodium is readily available and abundant, unlike lithium, there are fewer worries about resource scarcity and related expenses. Furthermore, because sodium-ion batteries make use of already-existing industrial processes, integrating them into production pipelines is simple and economical.
Concerns regarding resource scarcity and geopolitical dependencies are lessened by the fact that sodium, unlike lithium, can be mined from saltwater and abundant minerals.
A lower energy density and a shorter cycle life than their lithium-ion equivalents are two issues that SIBs must overcome despite their potential. To improve sodium-ion battery performance and stability, researchers are actively investigating new materials and electrode configurations.
The present state of Sodium Ion Battery technology, its fundamental ideas, its main benefits, and the continuous research efforts to get over current obstacles are all covered in this introduction.
Moreover, the adaptability of sodium-ion batteries allows for a wide range of applications in different industries. The cost and scalability of sodium-ion technology make it an attractive option for powering the upcoming generation of infrastructure and gadgets, ranging from electric vehicles and renewable energy storage systems to consumer electronics.
What is the working Sodium Ion Battery?
When the anode is charged, sodium ions go through the electrolyte from the cathode to the anode and embed themselves in the anode’s building blocks.
Discharging, on the other hand, produces electrical current that can be used to power external devices as the stored sodium ions are released from the anode and return to the cathode via the electrolyte.
The mechanism that allows sodium-ion batteries to store energy is the conversion of chemical energy into electrical energy while charging and vice versa when discharging.
What are the applications for Sodium Ion Battery ?
Sodium-ion batteries are intended for quick charge and discharge markets and can be used for a variety of purposes.
How do sodium-ion batteries compare to lithium ion batteries?
Sodium-ion batteries are safer than lithium-ion batteries and have a price advantage, but they have a lower energy density and are less efficient.
How long does a sodium ion battery last?
Sodium-ion batteries have a lifetime of 5000+ cycles, or 80% of their capacity.




